Akira Matsumoto
Cationic DABCO-Based Catalyst for Site-Selective C−H Alkylation via Photoinduced Hydrogen-Atom Transfer
A. Matsumoto, M. Yamamoto, K. Maruoka, ACS Catal. 12, 2045-2051 (2022).
Highlighted in Synfacts 2022, 18, 0423.
A series of hydrogen-atom transfer (HAT) catalysts based on the readily available and tunable 1,4-diazabicyclo[2.2.2]-octane (DABCO) structure was designed, and their photoinduced HAT catalysis ability was demonstrated. The combination of our HAT catalyst with an acridinium-based organophotoredox catalyst enables efficient and site-selective C−H alkylation of substrates ranging from unactivated hydrocarbons to complex molecules. Mechanistic studies suggested that the N-substituent of the catalyst plays a crucial role, assisting in the generation of a dicationic aminium radical as an active species for the HAT process.
Hyo-Jun Lee
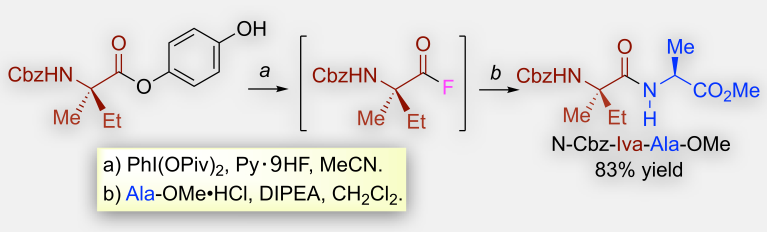
Metal-Free Approach for Hindered Amide-Bond Formation with Hypervalent Iodine(III) Reagents: Application to Hindered Peptide Synthesis

H.-J. Lee, X. Huang, S. Sakaki, K. Maruoka, Green Chem.23 848-845 (2020).
A new bio-inspired approach is reported for amide and peptide synthesis using α-amino esters that possess a potential activating group (PAG) at the ester residue. To activate the ester functionality under mild metal-free conditions, we exploited the facile dearomatization of phenols with hypervalent iodine(III) reagents. Using pyridine-hydrogen fluoride complex, highly reactive acyl fluoride intermediates can be successfully generated, thereby allowing for the smooth formation of sterically hindered amides and peptides from bulky amines and α-amino esters, respectively.
- Shunya Sakurai
- Akira Matsumoto
Cu-Catalyzed Enantioselective Alkylarylation of Vinylarenes Enabled by Chiral Binaphthyl-BOX Hybrid Ligands
S. Sakurai, A. Matsumoto, T. Kano, K. Maruoka, J. Am. Chem. Soc. 142, 19017-19022 (2020).
Transition-metal-catalyzed radical relay coupling reactions have recently emerged as one of the most powerful methods to achieve difunctionalization of olefins. However, there has been limited success in applying this method to asymmetric catalysis using effective chiral ligand. Reported is the Cu-catalyzed enantioselective alkylarylation of vinylarenes using alkylsilyl peroxides as alkyl radical sources by combining chiral bis(oxazoline) ligands with chiral binaphthyl scaffolds. The reaction affords chiral 1,1-diarylalkane structures that are found in a variety of bioactive molecules.
Zhe Wang
Efficient Cleavage of Tertiary Amide Bonds via Radical-Polar Crossover Using a Copper(II) Bromide/Selectfluor Hybrid System
Z. Wang, A. Matsumoto, K. Maruoka, Chem. Sci. 11, 12323-12328 (2020).
A novel approach for the efficient cleavage of the amide bonds in tertiary amides is reported. Based on the selective radical abstraction of a benzylic hydrogen atom by a CuBr2 /Selectfluor hybrid system followed by a selective cleavage of an N–C bond, an acyl fluoride intermediate is formed, and derivatized in a one-pot fashion. Mechanistic studies suggest that the reaction proceeds via a radical-polar crossover process. A synthetic application of this method for the selective cleavage of peptides is described.
Terumasa Kato
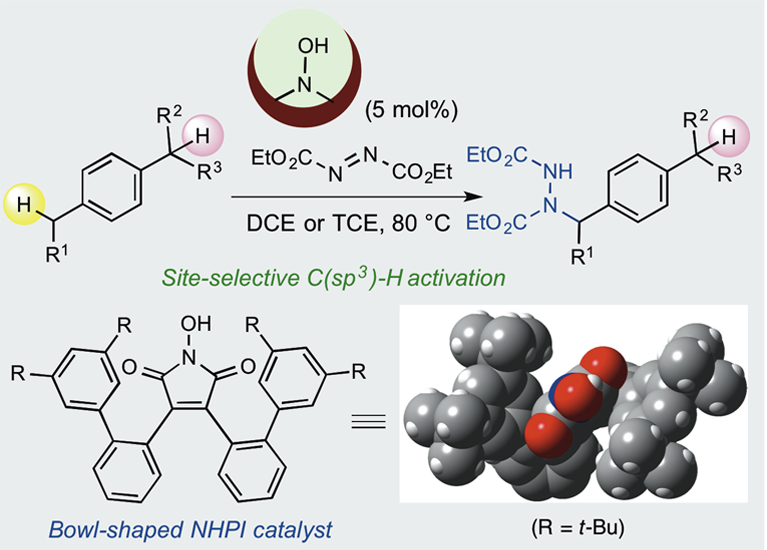
Design of Bowl-Shaped N-Hydroxyimide Derivatives as New Organoradical Catalysts for Site-Selective C(sp3)–H Bond Functionalization Reactions

T. Kato, K. Maruoka, Angew. Chem. Int. Ed. 59, 14261-14264 (2020).
Highlighted in Synfacts 2020, 16, 1212.
A series of new bowl-shaped N-hydroxyimide derivatives has been designed and used as selective organoradical catalysts. A number of these bowl-shaped N-hydroxyimide derivatives exhibit excellent site-selectivity in the amination of benzylic C(sp3)-H bonds in aromatic hydrocarbon substrates.
Akira Matsumoto
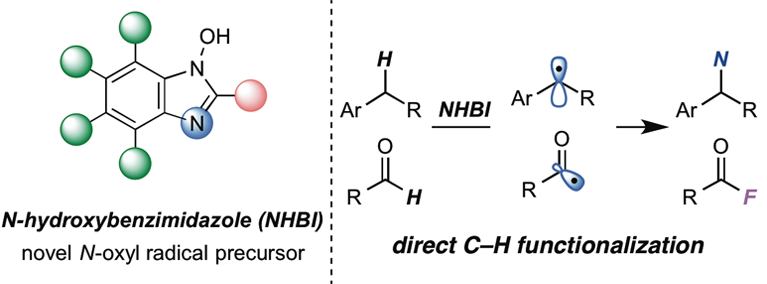
N-Hydroxybenzimidazole as a Structurally Modifiable Platform for N-Oxyl Radicals for Direct C–H Functionalization Reactions
T. Yoshii, S. Tsuzuki, S. Sakurai, R. Sakamoto, J. Jiang, M. Hatanaka, A. Matsumoto, K. Maruoka, Chem. Sci. 11, 5772-5778 (2020).
Methods for direct functionalization of C-H bonds mediated by N-oxyl radicals constitute a powerful tool in modern organic synthesis. While several N-oxyl radicals have been developed to date, the lack of structural diversity for these species has hampered further progress in this field. Here we designed a novel class of N-oxyl radicals based on N-hydroxybenzimidazole, and applied them to the direct C-H functionalization reactions. The flexibly modifiable features of these structures enabled facile tuning of their catalytic performance. Moreover, with these organoradicals, we have developed a metal-free approach for the synthesis of acyl fluorides via direct C-H fluorination of aldehydes under mild conditions.
Hanbin Lu (Alex)
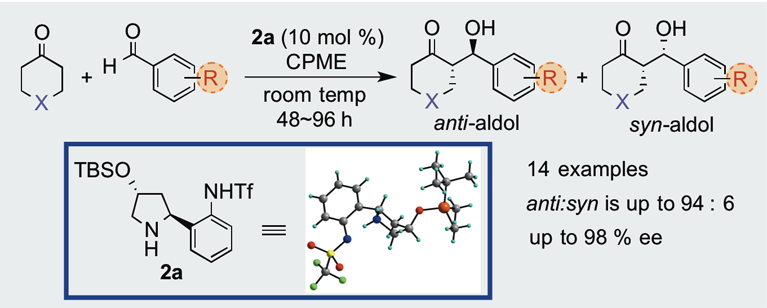
Practical Synthesis of High-Performance Amino Tf-Amide Organocatalysts for Asymmetric Aldol Reactions


H. Lu, J. Lv, C. Zhou, T. Kato, Y. Liu, K. Maruoka, Asian J. Org. Chem 9, 206-209 (2020).
A new type of optically pure secondary-amino aromatic Tfamide organocatalysts can be easily prepared from commercially available L-hydroxyproline. The chemical behavior of these organocatalysts was investigated by the application to asymmetric aldol reactions. Among these, catalyst 2a efficiently catalyzed direct asymmetric aldol reactions of cycloalkanones and substituted benzaldehydes, which afforded the corresponding aldol adducts in good yield with good to excellent anti-diastereo and enantioselectivity.
- Suva Paria
- Hyo-Jun Lee
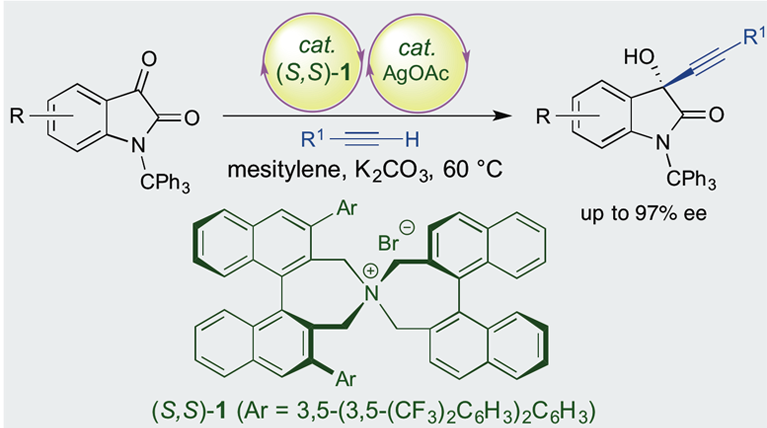
Enantioselective Alkynylation of Isatin Derivatives Using a Chiral Phase-Transfer/Transition-Metal Hybrid Catalyst System


S. Paria, H.-J. Lee, K. Maruoka, ACS Catal. 9, 2395-2399 (2019).
An enantioselective alkynylation of isatin derivatives can be realized by using a hybrid catalyst system consisting of chiral phase-transfer catalyst and transition metal catalyst. The chiral alkynylation products can be used as versatile intermediates for further synthetic transformations.