新規分泌性因子Neudesinの神経栄養・
分化因子としての役割の解明
遺伝子薬学分野 木村 郁夫
研 究 指 導 主 任 : 遺 伝 子 薬 学 分 野 教授 伊藤 信行
研究指導協力者 : 薬品作用解析学分野 教授 赤池 昭紀
研究内容
研究計画
近年、ヒトゲノムの解読が完了したが、その後の展開として各遺伝子の機能を探索することへと移行し、さらには遺伝子間、細胞間の相互作用ネットワークの解析へと展開することが予想される。その過程で新規有用遺伝子の発見はバイオ医薬や遺伝子治療等、医薬の面で非常に大きな影響を与えるものであると考えられる。そこでDNAデータベースより、分泌性シグナルを指標として、新規分泌性因子をコードすると予想され、機能不明なヒトおよびマウス遺伝子を見出し、後に神経栄養因子として作用することがわかったためにNeudesin と命名した。そしてこの遺伝子の成体における役割について検討した。
この新規分泌性因子Neudesinは胎児期から成体にかけて脳・脊髄等の中枢神経系において強く発現していた。また脳において神経細胞特異的に発現しており、神経細胞に対して生存維持活性を持つことを明らかにした。さらにこのNeudesinによる生存維持活性はMAPK, PI3K経路を介することによって発揮することを明らかにした。
今回、このNeudesinが神経分化時期の mouse脳に強く発現していることおよび培養神経系前駆細胞においても発現していることから神経系前駆細胞における神経分化に対するNeudesinの役割について検討を行う。 培養神経系前駆細胞を用いたNeudesin添加実験を行い、分化後の細胞運命を免疫染色により染め分けることで神経分化へのNeudesinの影響について検討を行う。また同時に神経分化に関与する種々の転写因子、細胞外成長因子等のNeudesin添加による発現変化をRT-PCRによって検討する。さらにその時の細胞内シグナル伝達をそれぞれの経路の阻害剤やリン酸化抗体を用い検討する。これらの結果からNeudesinの作用する受容体についての予測をたてる。
以上を明らかにすることにより神経細胞に対する生存維持活性だけではなく神経分化、神経再生医療の観点からも創薬応用できる非常に有用な因子であることが期待できる。また受容体を予測することで創薬ターゲットとして現実的応用の可能性が期待できる。これらよりNeudesinの研究は神経分化、再生、脳・神経系疾患に対する予防・治療薬の開発応用に対し、有用な知見を提供するものと期待される。
Summary
Neudesin expressed in adult mouse brain encodes a secreted signal with neurotrophic activity in neurons (J Neurosci Res. 79:287, 2005). Most neurotrophic factors are involved in neural cell proliferation and/or differentiation. However, the role of Neudesin in neural development remains to be elucidated. Therefore, we examined the expression of Neudesin in mouse embryonic cerebral cortex and cultured mouse neural precursor cells and its roles in neural development. Neudesin was expressed in the embryonic cerebral cortex early in development. Its expression was mainly observed in the preplate where mostly post-mitotic neural cells existed. Neudesin mRNA was expressed in the neural precursor cells before the appearance of neurons. Therefore, roles of Neudesin in neural development were examined using the precursor cells. Neudesin significantly promoted neuronal differentiation and overrode the undifferentiated state of the neural precursor cells sustained by FGF2. In contrast, it inhibited the differentiation of astrocytes. In addition, Neudesin transiently promoted neural cell proliferation early in the developmental process. The effect on cell proliferation was distinct from that of FGF2, a self-renewal-promoting factor for neural precursor cells. The differentiation was mediated though activation of the protein kinase A (PKA) and phosphatidylinositol-3 kinase (PI-3K) pathways. In contrast, the proliferation was mediated through the mitogen-activated protein kinase and PKA pathways. The expression profile and activity indicate that Neudesin plays unique roles in neural development. The present findings have revealed new potential roles of Neudesin in neural cell proliferation and neuronal differentiation.
要旨
以前、我々が報告した新規神経栄養因子Neudesin (J Neurosci Res. 79:287, 2005)が神経系前駆細胞において一過性の増殖後、神経細胞への分化を促進する因子であることを明らかにした。
mouse Neudesinが神経分化期において大脳皮質に強く発現していることから培養神経系前駆細胞を用い、神経分化への影響についての検討を行った。その結果、Neudesinは神経系前駆細胞の増殖を一時的に促進させ、その増殖活性はPKA経路に依存するMAPK経路を介するものであることがわかった。さらにこの一過性の増殖後、神経細胞分化に関与する転写因子の上昇が確認され、PI3K、PKA経路を介してNeudesinが神経細胞分化を促進することがわかった。またこの時、PKA経路によりBDNFが誘導され、これが神経分化を促進する可能性も示唆された。さらにNeudesinのアストロサイトへの分化に対する影響を検討したところ、Neudesinはその分化を抑制することもわかった。
これらの結果より、Neudesinは選択的に神経細胞分化を促進する重要な因子であることが示唆された。
Fig.1 Expression of Neudesin in mouse embryonic cerebral cortex and Neural precursor cells.
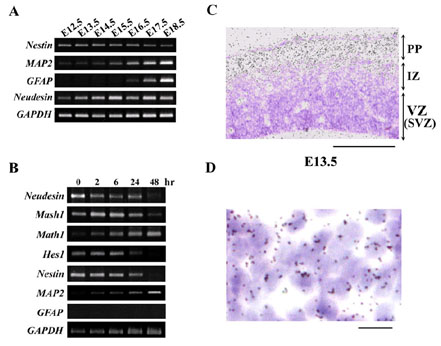
A: The expression of Neudesin and marker genes for neural cells was examined in mouse embryonic cerebral cortex at E12.5-E18.5 by RT-PCR.
B: The expression of Neudesin and marker genes for neural cells was examined in mouse neural precursor cells by RT-PCR.
C: The expression of Neudesin was examined in mouse cerebral cortex at E13.5 by in situ hybridization using 35S-labeled antisense Neudesin cRNA. The section was counterstained with hematoxlin-eosin. Black grains in a bright-field photograph indicate the localization of Neudesin mRNA. PP, the preplate; IZ, the intermediate zone; VZ, the ventricular zone; SVZ, subventricular zone. Scale bar = 200mm.
D: Cellular localization of Neudesin in the preplate examined by in situ hybridization was shown at a higher magnification. Black grains in a bright-field photograph indicate the localization of Neudesin mRNA. Scale bar = 10mm.
Fig.2 Neudesin induces neuronal differentiation related transcription factors and growth factors.
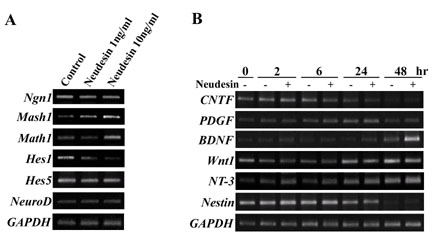
A: RT-PCR analysis for expression of neuronal differentiation related transcription factors in mouse neural precursor cells treated with recombinant Neudesin protein for 24hr.
B: RT-PCR analysis for expression of neural differentiation related growth factors. Mouse neural precursor cells cultured were treated for 0-48hr with or without Neudesin (1ng/ml). GAPDH were used as a loading control.
Fig.3 Effects of Neudesin on neuronal and astrocyte differentiation in mouse neural precursor cells.
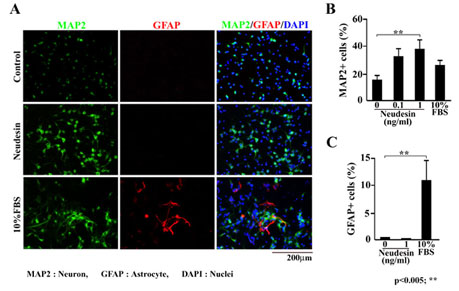
A: Mouse neural precursor cells were cultured in the presence of Neudesin (0.1-1ng/ml) or FBS (10%). After cultured for 3days, the neural precursor cells were double-stained with anti-MAP2 and GFAP antibodies. Green and red signals indicate MAP2 and GFAP-positive cells, respectively. Blue signals indicate cell nuclei counterstained with DAPI. Scale bar = 200mm.
B: The effect of Neudesin on neuronal differentiation was quantitatively determined by counting MAP2-positive cells.
C: The effect of Neudesin on astrocyte differentiation was quantitatively determined by counting GFAP-positive cells. Results are means ± SEM of five different fields from four independent slides. **P < 0.005.
Fig.4 Effects of Neudesin on neuronal differentiation inhibited by FGF2 in mouse neural precursor cells.
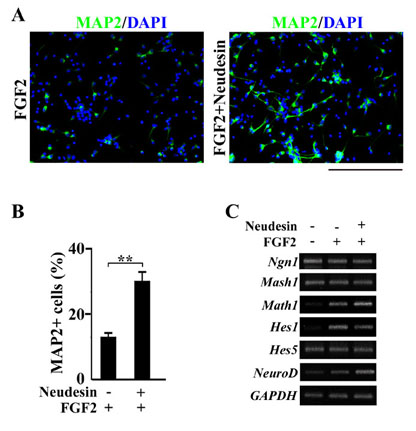
A: Mouse neural precursor cells were cultured in the presence of FGF2 (10ng/ml) and Neudesin (0 or 1ng/ml). After cultured for 4days, the neural precursor cells were examined by immunofluorescence analysis using anti-MAP2 antibody. Green signals indicate MAP2-positive cells. Blue signals indicate cell nuclei counterstained with DAPI. Scale bar = 200mm.
B: The effect of Neudesin (1ng/ml) on neuronal differentiation repressed by FGF2 (10ng/ml) was quantitatively determined by counting MAP2-positive cells. Results are means ± SEM of five different fields from four independent slides. **P < 0.005.
C: RT-PCR analysis for expression of neuronal differentiation related transcription factors, treated with Neudesin. Mouse neural precursor cells cultured in the presence of FGF2 (10ng/ml) were treated with recombinant Neudesin protein for 24hr.
Fig.5 Effects of Neudesin on astrocyte differentiation induced by FBS in mouse neural precursor cells.
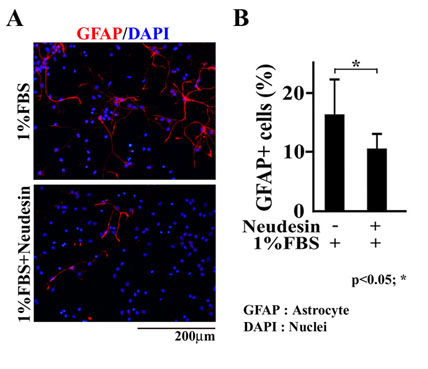
A: After being cultured for 2hr, the neural precursor cells were cultured in the presence of FBS (1%) and Neudesin (0 or 1ng/ml). After being cultured for 7days, the neural precursor cells were examined by immunofluorescence analysis using anti-GFAP antibody. Red signals indicate GFAP-positive cells. Blue signals indicate cell nuclei counterstained with DAPI.
B: The effect of Neudesin (1ng/ml) on astrocyte differentiation promoted by FBS (1%) was quantitatively determined by counting GFAP-positive cells. Results are means ± SEM of five different fields from four independent slides. *P < 0.05. Scale bar = 200mm.
Fig.6 Effects of Neudesin on phosphorylation of ERK1/2, Akt and CREB in mouse neural precursor cells.
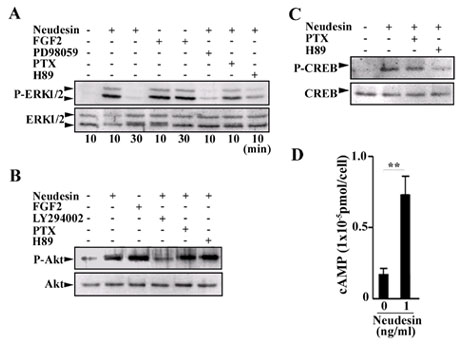
A, B, C: After being cultured for 12hr, the neural precursor cells were further cultured for 10 or 30min in the presence or absence of Neudesin (1ng/ml). Inhibitor, PD98059 (60mM), H89 (10mM), LY294002 (40mM), PTX (100ng/ml) were added to the medium at 2hr before the addition of Neudesin. ERK1/2, Akt, CREB and their phosphorylated forms were detected by Western blotting using their antibodies.
D: After being cultured for 14hr, the neural precursor cells pre-cultured with IBMX for 10min were cultured in the presence of Neudesin (0 or 1ng/ml) for 5min. The cAMP levels in the cells were determined using a cAMP-EIA kit. Results are means ± SEM of four independent wells. **P < 0.005.
Fig.7 Effects of Neudesin on neural cell proliferation in mouse Neural precursor cells.
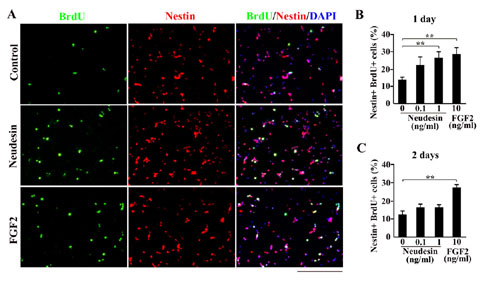
A: After cultured for 2hr, the neural precursor cells were further cultured in the presence of BrdU and Neudesin (0.1-1ng/ml) or FGF2 (10ng/ml). After cultured for 22hr, the neural precursor cells were examined by double-stained with anti-BrdU and Nestin-positive cells, respectively. Blue signals indicate cell nuclei counterstained with DAPI. Scale bar = 200mm.
B: The effect of Neudesin on neural cell proliferation was quantitatively determined by counting BrdU and Nestin-double positive cells.
C: After pre-cultured for 24hr in the presence of Neudesin or FGF2, the neural precursor cells were further cultured for 24hr in the presence of BrdU and Neudesin or FGF2. The effect of Neudesin on neural cell proliferation was quantitatively determined by counting BrdU and Nestin-double positive cells. Results are means ± SEM of five different fields from four independent slides. **P < 0.005.
Fig.8 Effects of inhibitors for PI-3K and PKA pathways on neuronal Differentiation activity promoted by Neudesin in mouse neural precursor cells.
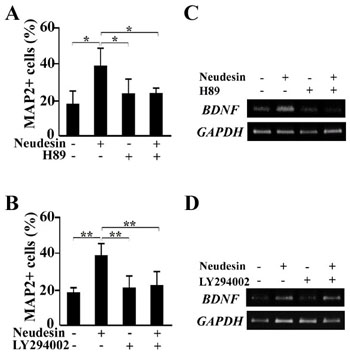
A, B: After cultured for 12hr, the neural precursor cells were further cultured in the presence of Neudesin (0 or 1ng/ml). Inhibitors, H89 (10mM) (A) and LY294002 (40mM) (B) were added to the medium at 2hr before the addition of Neudesin. After cultured for 3days, the neural precursor cells were examined by immunochemistry using anti-MAP2 antibody. The effect of the inhibitors on neuronal differentiation promoted by Neudesin was quantitatively determined by counting MAP2-positive cells. Results are means ± SEM of five different fields from four independent slides. *P < 0.05; **P < 0.005.
C, D: RT-PCR analysis for expression of induced BDNF by Neudesin. Mouse neural precursor cells were trested with Neudesin (1ng/ml) and inhibitor (H89; C, LY294002; D) for 48hr. GAPDH was used as a loading control.
Fig.9 Effects of inhibitors for MAPK, PKA and PI-3K pathways on neural cell proliferation promoted by Neudesin in mouse neural precursor cells.
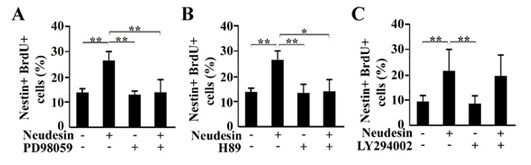
After cultured for 2hr, the neural precursor cells were further cultured in the presence of BrdU and Neudesin (0 or 1ng/ml). Inhibitors, LY294002 (40mM) (A), H89 (10mM) (B) and PD98059 (60mM) (C) were added to the medium at 2hr before addition of Neudesin. After cultured for 22hr, the neural precursor cells were examined by double immunochemistry using anti-BrdU and Nestin antibodies. The effect of Neudesin on neural cell proliferation was quantitatively determined by counting BrdU and Nestin-double positive cells. Results are means ± SEM of five different fields from four independent slides. *P < 0.05; **P < 0.005.
Putative Neudesin signal pathways and neural differentiation model.
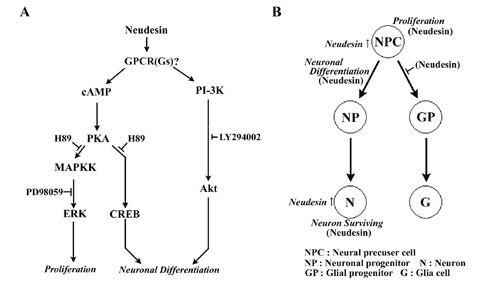
|

