丂揤慠偵懚嵼偡傞懡偔偺僞儞僷僋幙偼丄暋嶨側攝楍傪傕偮摐嵔偵傛偭偰擹枾偵廋忺偝傟偰偍傝丄嬤擭丄偙傟傜偺摐嵔偼妀巁丄傾儈僲巁偵師偖戞俁偺惗柦忣曬嵔偲偟偰偦偺婡擻夝愅偵拲栚偑偁傃偮偮偁傞丅杮尋媶偱偼丄摐嵔偵撪嵼偝傟傞惗懱忣曬偺夝撉傪栚揑偲偟丄俵俛俹乮mannan binding protein乯偑惗懱撪偱壥偨偡栶妱偺夝柧傪傔偞偟偰偄傞丅俵俛俹偼儅儞僲乕僗丄僼僐乕僗丄俶-傾僙僠儖僌儖僐僒儈儞傪摿堎揑偵擣幆偡傞俠宆儗僋僠儞偺堦庬偱偁傞丅俵俛俹偼堦庬偺堚揱巕偵僐乕僪偝傟丄庬偵娞憻偱崌惉偝傟傞偑丄嵶朎奜偵暘斿偝傟傞寣惔宆俵俛俹乮俽-俵俛俹乯偲嵶朎撪偵棷傑傞嵶朎撪俵俛俹乮俬-俵俛俹乯偑懚嵼偡傞丅俽-俵俛俹偼愭揤惈柶塽偵偍偄偰廳梫側暘巕偱偁傞偑丄俬-俵俛俹偺婡擻偵偮偄偰偼枹偩偵晄柧側揰偑懡偄丅偙傟傑偱偺尋媶偵傛傝丄嫟徟揰尠旝嬀傗揹巕尠旝嬀傪梡偄偨娤嶡偐傜丄俬-俵俛俹偑彫朎懱乮俤俼乯偺堦晹偵摿挜揑側懚嵼條幃傪帵偡偙偲偐傜柧傜偐偲偟偨丅偝傜偵俤俼偲俧倧倢倗倝偺憡揷傪峴偒棃偡傞桝憲彫朎偺僞儞僷僋幙儅乕僇乕偲斾妑丄娤嶡偟偨寢壥偐傜丄俬-俵俛俹偼偦傟傜桝憲彫朎拞偵懚嵼偡傞偙偲偑柧傜偐偲側傝丄摐僞儞僷僋幙偺嵶朎撪桝憲偵娭梌偡傞壜擻惈傪帵偟偨丅崱屻丄嫟徟揰尠旝嬀丄揹巕尠旝嬀傪梡偄偰偝傜側傞徻嵶側娤嶡傪峴偄丄嵶朎撪偱俵俛俹偑偳偺桝憲彫朎偵嬊嵼偟偰偄傞偐傪挷傋丄傑偨俽-俵俛俹偲俬-俵俛俹偺東栿屻廋忺丄暘斿偵偳偺傛偆側堘偄偑偁傞偐傪柧傜偐偲偡傞丅師偵丄俬-俵俛俹偲嵶朎撪偵偍偄偰憡屳嶌梡偡傞僞儞僷僋幙偺摨掕傪峴偆丅偝傜偵俬-俵俛俹偲偺寢崌丄夝棧偺儊僇僯僘儉側偳偵偮偄偰昞柧僾儔僘儌儞嫟柭朄傪梡偄偨徻嵶側夝愅傪峴偆偙偲偵傛傝丄俬-俵俛俹偑嵶朎撪偺娐嫬偵偍偄偰丄怴惗摐僞儞僷僋幙偲寢崌丄夝棧傪偡傞偙偲傪帵偡丅偙傟傜偺尋媶偵傛傝丄俬-俵俛俹偑扴偆摐僞儞僷僋幙偺昳幙娗棟偵偍偗傞栶妱偑柧妋偵側傞偲峫偊傜傟傞丅杮尋媶悑峴偵偼怽惪幰偑強懏偡傞尋媶幒偺帩偮摐嵔惗暔妛揑尋媶庤朄偩偗偱偼廫暘偱偼側偔丄嵶朎撪僞儞僷僋幙偺慖戰揑桝憲婡峔偵娭偡傞愱栧揑側嵶朎惗暔妛揑抦幆偲宱尡偑昁梫偱偁傞丅廬偭偰杮尋媶偼屄乆偺尋媶暘栰偺妋棫偟偰偄傞抦尒傗媄弍偺梈崌偵傛偭偰払惉壜擻側尋媶偱偁傝丄俬-俵俛俹偵娭偡傞怴偟偄婡擻夝柧偑婜懸偝傟傞丅
- Subcellular Localization of I-MBP in ER and Golgi
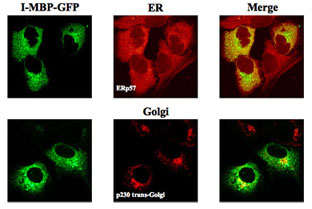
The subcellular distribution and co-localization of I-MBP with the ER and Golgi apparatus was visualized by confocal laser scan microscopy.The HuH-7 MBP-GFP stable transfectants were stained with ER marker, ERp57 and Golgi marker, p230 trans-Golgi, respectively, both followed by the corresponding secondary antibodies labeled with Alexa Fluor 568.
- Electron Microscopy Analysis for Subcelluar Localization of I-MBP
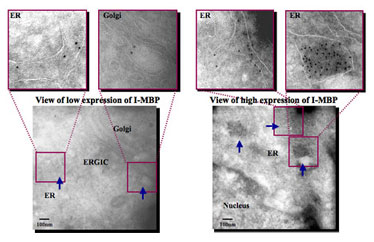
The pEGFP-N1-MBP-transfected HeLa cells were fixed and embedded.Ultrathin cryosections of the HeLa cells were stained with anti-GFP pAb, followed with 10nm colloidal immunogold labeled secondary antibody.The arrows indicate the accumlative of I-MBP in the ER, Golgi and ERGIC.
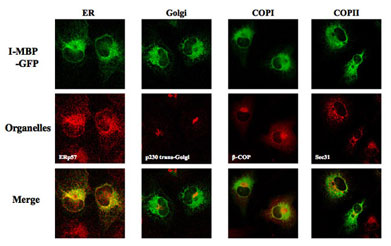
- Functional Localization of I-MBP in COP嘥 and COP嘦 Vesicles
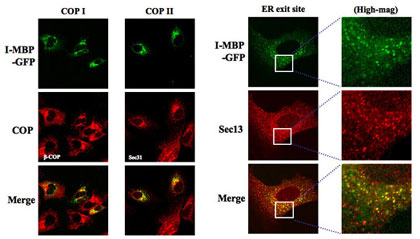
HuH-7 cells staby expressing MBP-GFP were stained with COP嘥 marker, β-COP, COP嘦 marker, Sec 31A, and ER exit site marker of COP嘦, Sec 13, all followed by the secondary antibodies labeled with Alexa Fluor 568, and then analyzed by confocal laser scan microscopy.
- Newly Synthesized LAMP-1 Undergoes Interaction with I-MBP and BIP
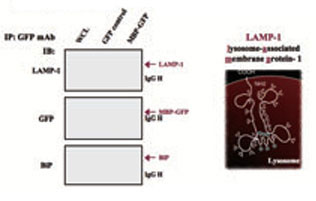
HEK293 cells were ransfected with pEGFP-N1 or pEGFP-N1-MBP vector. After 24h posttransfection, the transfectants were crosslinked with crosslinker 1 mM DSP, and the whole cell lysates(WCL) of transfectants were then immunoprecipitated by anti-GFP mAb, The immunoblots were probed with antibodies to LAMP-1, I-MBP-GFP and BIP, respectively.
- The Ca2+ Requirement for Binding of I-MBP to LAMP-1 in vivo
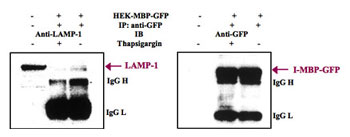
HEK293 cells transfected with pEGFP-N1 of pEGFP-N1-MBP were incubated for 12 h in the absence or presence of ER Ca+-ATPase inhibitor, 1 µM thapsigargin, and then incubated for 30 min with 1 mM DSP for crosslinking.The whole cell lysates were immunoprecipitated by anti-GFP mAb, and immunoblots were probed with antibodies to LAMP-1 and I-MBP-GFP, respectively.
- Effects of N-glycosylation Inhibitor Treatment on I-MBP / LAMP-1 Interation
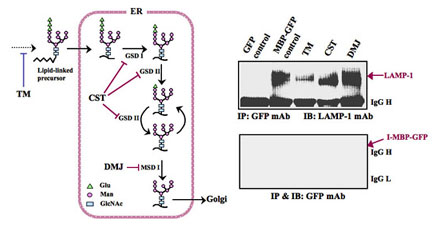
HEK293 cells transiently transfected with pEGFP-N1 or pEGFP-N1-MBP were incubated for 24 h in the absence or presence of N-glycosylation inhibitors, 2 µg/ml TM, 1 mM DMJ, respectively, and then incubated for 30 min 1 mM DSP in crosslinking buffer on a rocking platform.The immunoprecipitates with anti-GFP mAb were probed with antibodies to LAMP-1 and I-MBP-GFP, respectively.
TM, tunicamycin; CST, castanosermine; DMJ, deoxymannojirimycin;Glu, glucose; Man, mannnose; GlcNAc, N-acetylglucosamine.
- Carbohydrate-binding Assay of Wild-type and Mutant I-MBPs
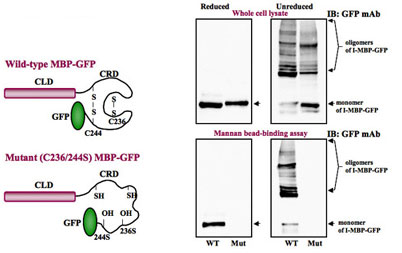
The wild-type or mutant I-MBP-GFP extracts from stable transfected HLF cells were incubated with mannan-sepharose 4B beads in the presence of 5mM CaCl2, and the bound proteins were eluted with elution buffer containing 4mM EDTA. The eluted proteins were separated by SDS-PAGE under reduced conditions, and immunoblots were probed with antibodies to I-MBP-GFP, respectively.
- Subcellular Localization of Mut I-MBP in ER but not in Golgi, COP嘥 and COP嘦 Vesicles
The HLF cells stably transfected with pEGFP-N1-Mutant MBP vector were stained with ER marker, ERp57, Golgi marker, p230 trans-Golgi, COP嘥 marker, β-COP and COP嘦 marker, Sec 31A, respectively, all followed by the corresponding secondary antibodies labeled with Alexa Fluor 568.
- Characterization of Biosynthesis and Differentiation of S-MBP and I-MBP in Human Hepatoma Cells
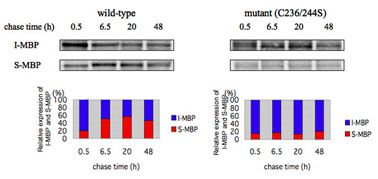
HLF cells stably expressing wild-type and mutant(C236/244S) MBP-GFPs were pulse-labeled with [35S]Met and [35S]Cys for 1 h, and were then washed and chased for up to 48 h as indicated in the figure.The medium was collected and concentrated, and the cells were solubilized with 1% NP40 lysis buffer.The immunoprecipitated S- and I-MBP-GFP with anti-GFP mAb were resolved on SDS-PAGE under reduced conditions followed by transfer to a nitrocellulose membrane, and then S-MBP and I-MBP signals of wild-type and mutant(C236/244S) on fluorograms were quantified by densitometry as shown in upper panels.The relative levels of I-MBP and S-MBP were determined comparing the expressions between I-MBP and S-MBP at te indicated chase times as shown in lower panels.
- Ultracentrifugation Analysis for Subcellular Fractions of Wild-type and Mutant I-MBPs
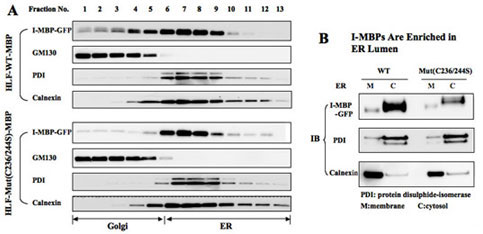
俙丏HLF cells stably expressing wild-type and mutant(C236/244S) MBP-GFPs were homogenized, and the post-nuclear supernatans of centrifugation onto 5-30% linear OptiPrep gradient were separated by ultracetrifugation, respectively, An aliquot of each fraction was analyzed by SDS-PAGE, and the distribution of I-MBP and organelle marker proteins was determined by direct immunoblotting.
俛丏ER fractions(No.6-10丠) of wild-type and mutant I-MBP-GFPs were collected and incubated with 0.1 M Na2CO3, pH 11.0 to extract soluble proteins from the lumen before collecting membrances followed by centrifugation. The pallet and soluble fractions were analyzed by immunoblotting using GFP, PDI and calnexin antibodies.

