Research
Functional Analysis of G-Protein-Coupled Receptors (GPCRs)
Research Overview
G-protein-coupled receptors (GPCRs) are seven-transmembrane receptors located on the cell surface that function as sensors for a wide variety of extracellular substances, including hormones, peptides, and bioactive amines. Among the approximately 800 GPCRs present in the human genome, more than 100 serve as drug targets, with nearly 500 therapeutic agents approved for these GPCRs. This represents about 30% of all currently approved drugs, making GPCRs important drug targets.
Our laboratory aims to understand the complex intracellular signaling mechanisms of GPCRs in detail through the development of novel activity detection methods for signaling molecules and the establishment of signaling molecule-deficient cells. The ultimate goal of this research is to identify new drug targets and create superior therapeutic agents.
Major Research Themes
Research Theme List
GPCR Intracellular Signaling

GPCRs activated by ligand binding activate various intracellular signaling molecules. The major GPCR signaling molecules known include heterotrimeric G proteins, GPCR kinases (GRK), and β-arrestins. GPCRs activate intracellular heterotrimeric G proteins and are then phosphorylated by GRK. Phosphorylated GPCRs are recognized by β-arrestins and sequestered into endosomes.
Depending on the combination of GPCR and ligand, the types of activated heterotrimeric G proteins and the phosphorylation sites by GRK differ. Furthermore, recent reports show that β-arrestin also functions as a signaling molecule, making GPCR intracellular signaling extremely complex.
Development of GPCR Signal Activation Detection Method Using TGFα Cleavage
Heterotrimeric G proteins activated by GPCRs are broadly classified into Gs, Gi, Gq, and G12 based on their functions. Among these, Gs, Gi, and Gq are classical heterotrimeric G proteins, and their activation can be easily measured because suitable second messengers exist for detection and are well-analyzed. In contrast, G12 signaling acts on actin cytoskeleton regulation, making activation measurement difficult due to the lack of suitable second messengers.


Our laboratory discovered that TGFα present on the cell membrane is cleaved and released extracellularly downstream of Gq or G12 signaling during functional analysis of GPCRs in hair follicles. By applying this reaction, we established an experimental system that can detect Gq or G12 activation with high throughput and sensitivity. Furthermore, by improving this experimental system using Gq and G12 knockout cells created with the CRISPR-Cas9 method and chimeric Gα proteins, we made it possible to detect activation of Gs, Gi, Gq, and G12 on the same platform. This experimental system is called the TGFα cleavage assay and is used as a GPCR activation detection method in numerous laboratories both domestically and internationally (Inoue A et al., Nature Methods, 2012).
We also found that this method can detect activation not only of GPCRs but also of some ion channels, demonstrating its utility for detecting activation of a wide range of membrane proteins.
Construction and Application of GPCR-G Protein Coupling Database
Using the TGFα cleavage assay mentioned above, we comprehensively evaluated the coupling between 148 GPCRs with known ligands and four types of heterotrimeric G proteins, including G12, which had not been extensively evaluated (Inoue A et al., Cell, 2019). As a result, we newly discovered that more than 50 GPCRs couple with G12. Furthermore, by analyzing this GPCR-G protein coupling database using bioinformatics methods, we constructed a program (PRECOG, http://precog.russelllab.org) that predicts coupling heterotrimeric G proteins based on GPCR amino acid sequences (Singh G et at., Nucleic Acid Res, 2019).

Functional Analysis of G12 Signaling Using G12-Coupled Designer GPCRs

Recently, artificial GPCRs called designer GPCRs have attracted attention as useful tools for GPCR functional analysis. Designer GPCRs, also known as DREADDs (Designer Receptors Exclusively Activated by Designer Drug), do not respond to endogenous ligands and are activated by specific artificial ligands. The commonly used designer GPCR and designer ligand are acetylcholine receptor variants and CNO (clozapine N-oxide).
Previously, DREADDs that selectively couple to Gs, Gi, and Gq had been reported, but no DREADD coupling to G12 had been reported. Using PRECOG mentioned above, we searched for amino acid sequences favoring coupling with G12 and introduced them into Gq-coupled DREADDs, successfully creating the world's first G12-coupled DREADD (Inoue A et al., Cell, 2019).
Using the TGFα cleavage assay, we newly discovered that more than 50 GPCRs couple with G12, but the functions of these GPCRs mediated by G12 are largely unknown. The G12-coupled DREADD is a research tool that can activate G12 signaling under synthetic ligand control at the individual level and can overcome this problem. Our laboratory has created mice that express G12-coupled DREADDs in a Cre recombinase-dependent manner and is advancing functional analysis of G12 at the individual level.
Development of GPCR Signal Activation Detection Method Using Split Luciferase
GPCR signaling, after being activated by ligands, undergoes dynamic signal transduction within cells. Previous analysis methods often evaluated signal accumulation at specific time points, making it difficult to track the temporal behavior of signaling molecules. We have established methods to evaluate the following temporal changes by using the high-brightness split luciferase NanoBiT system to assess interactions between two proteins:
• Protein dissociation (dissociation of Gα and Gβγ subunits)
• Binding of Gα proteins and effector proteins
• Binding of GPCR-GRK and GPCR-β-arrestin
Computational Analysis of GPCR Activation Mechanisms
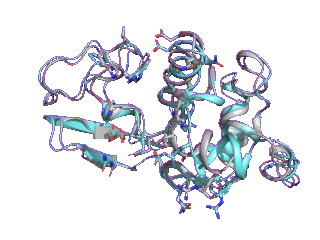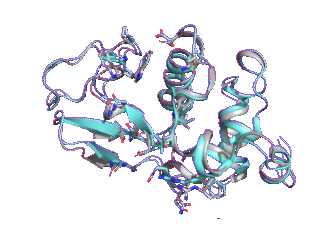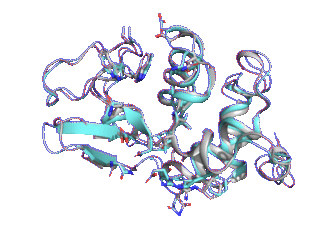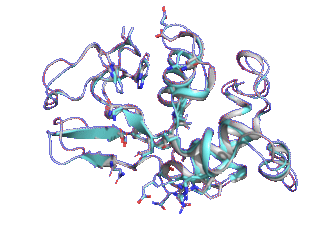
Proteins such as GPCRs undergo conformational changes by interacting with other molecules such as ligands and substrates, exhibiting functions corresponding to their shapes. Although many three-dimensional structures of GPCRs in active and inactive states have been determined, it is difficult to elucidate from static protein structures alone what conformational change processes inactive proteins undergo to reach the active state and which amino acid residues trigger these changes.
Therefore, we are trying to elucidate the dynamic properties of GPCRs by observing protein movements at the atomic level using a method called molecular dynamics simulation.
Understanding GPCR Signaling Using GPCR Effector Protein-Deficient Cells
Our laboratory has created cells deficient in proteins that function directly downstream of GPCRs in HEK293 cells, which are commonly used in GPCR signaling research, and applies them to research. Specifically, we use them for functional analysis of disease-causing mutations and analysis of how multiple signals interact with each other (signal crosstalk). We currently possess the above cell panel and can provide it for distribution.
Single-Molecule Imaging of GPCR-Effector Interactions in Living Cells
"When," "where," and "how" GPCRs and effectors interact are important questions for understanding intracellular signal transduction. We have developed a fluorescence microscopy system that can measure the movement, association state, and binding/dissociation of GPCR-effector molecules at the single-molecule level in living cells, and are working to elucidate the spatial control mechanisms of signal transduction.
By observing single molecules, we can see that GPCR molecules in the same cell membrane also have "individuality." Cells are expected to achieve highly efficient information processing by constructing workplaces where GPCRs and effector molecules are concentrated in the plasma membrane. In the future, we will analyze ligand-stimulation-dependent dynamic changes of GPCR and effector molecules to elucidate the mechanisms of action of GPCR-targeted drugs.